El objetivo es conocer las pilas de combustible de metanol dmfc sus fundamentos y las ventajas y las limitaciones de su uso respecto a otras.
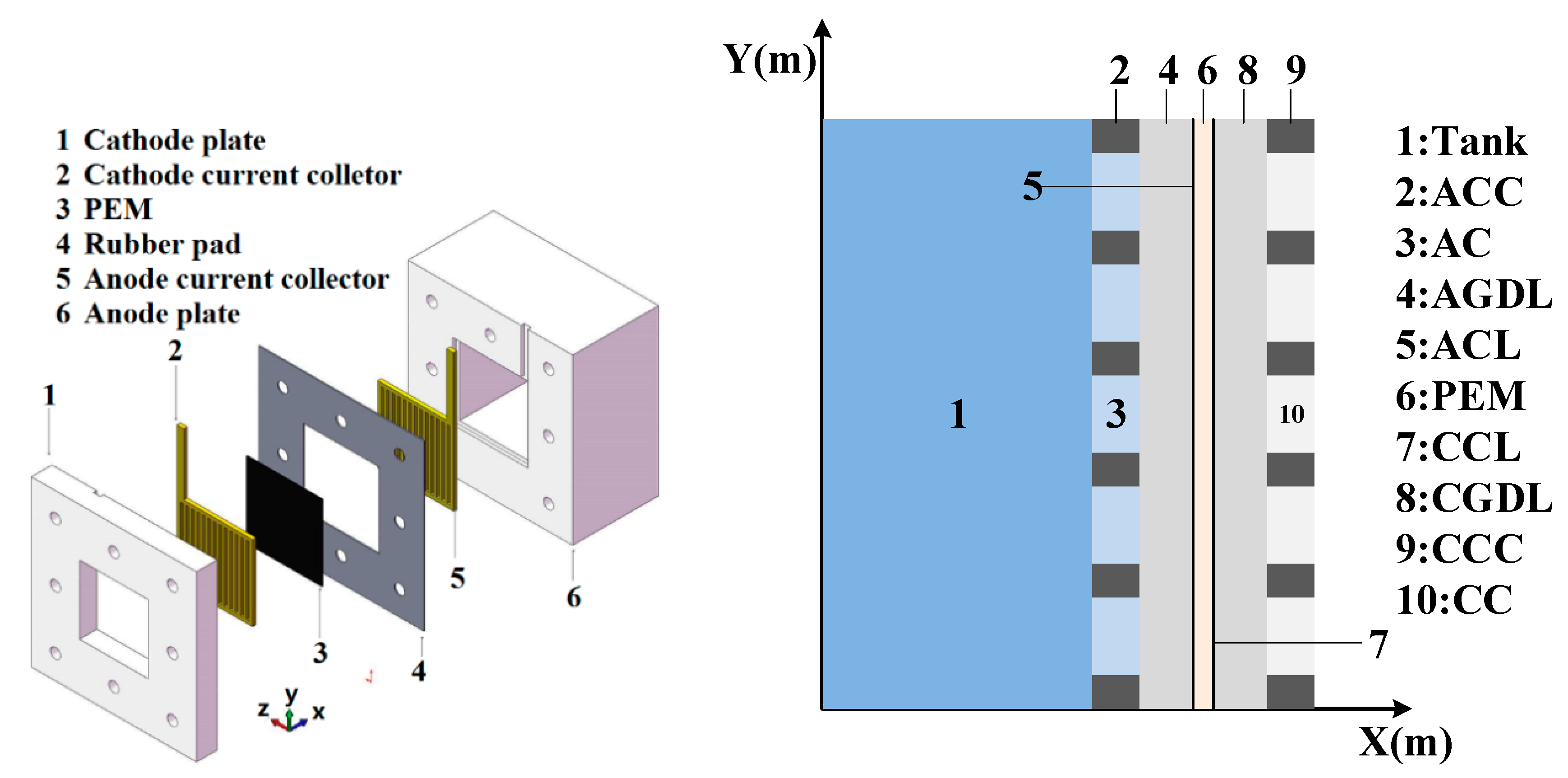
Direct methanol fuel cell working principle.
Direct methanol fuel cells descripción.
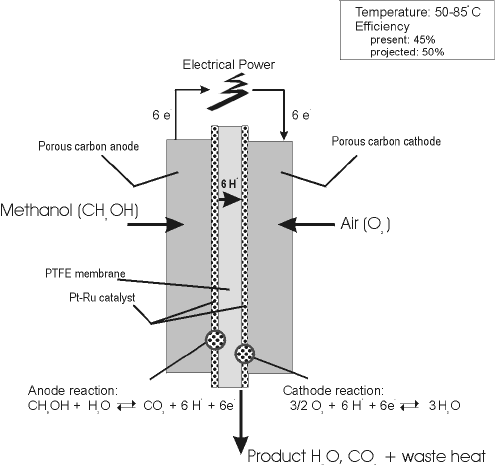
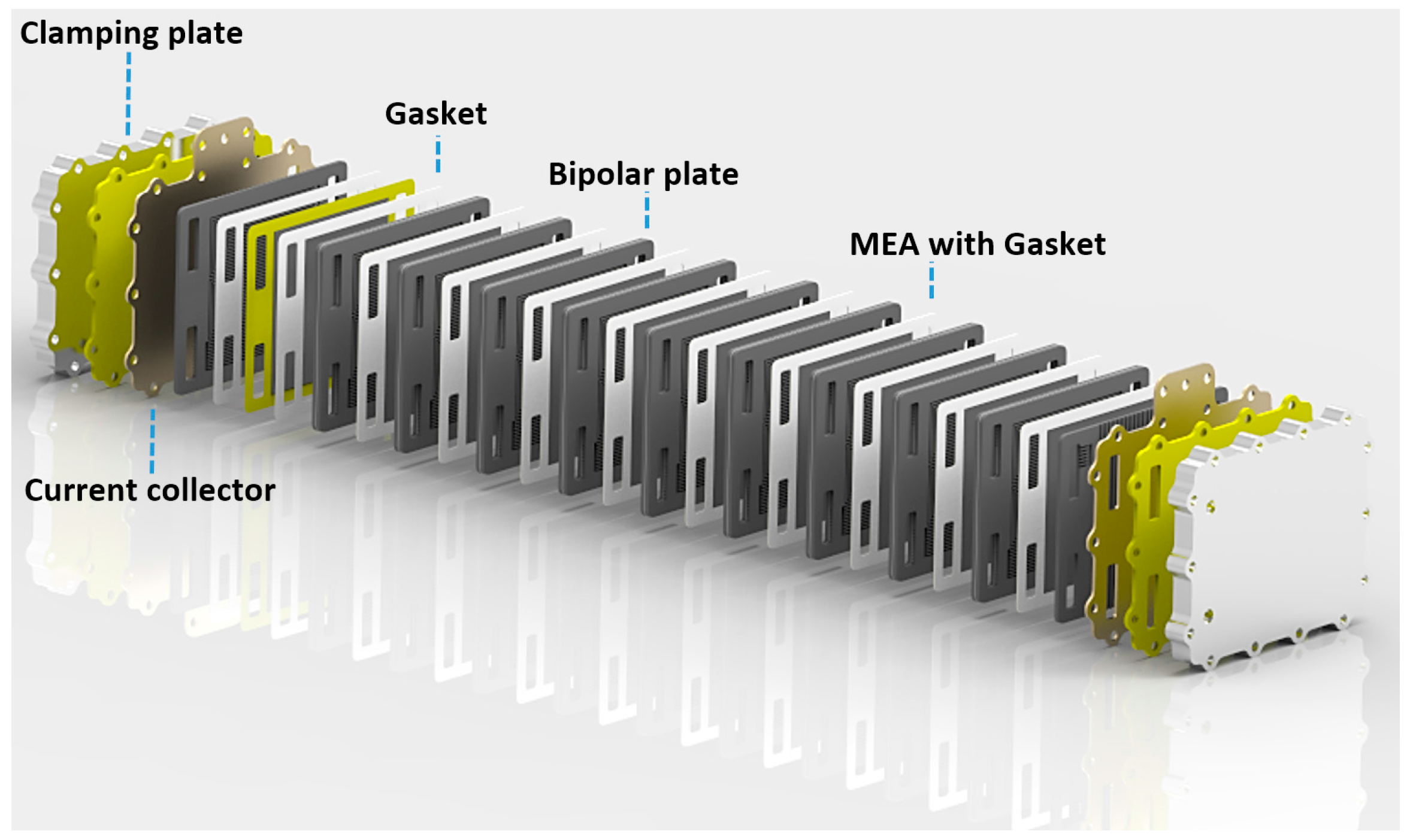
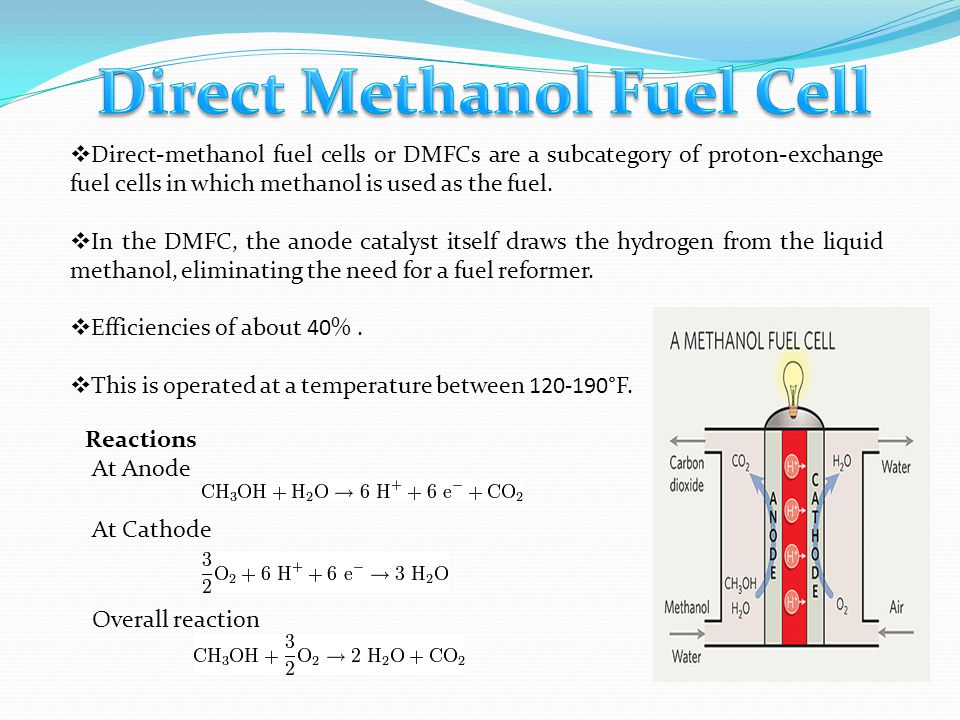
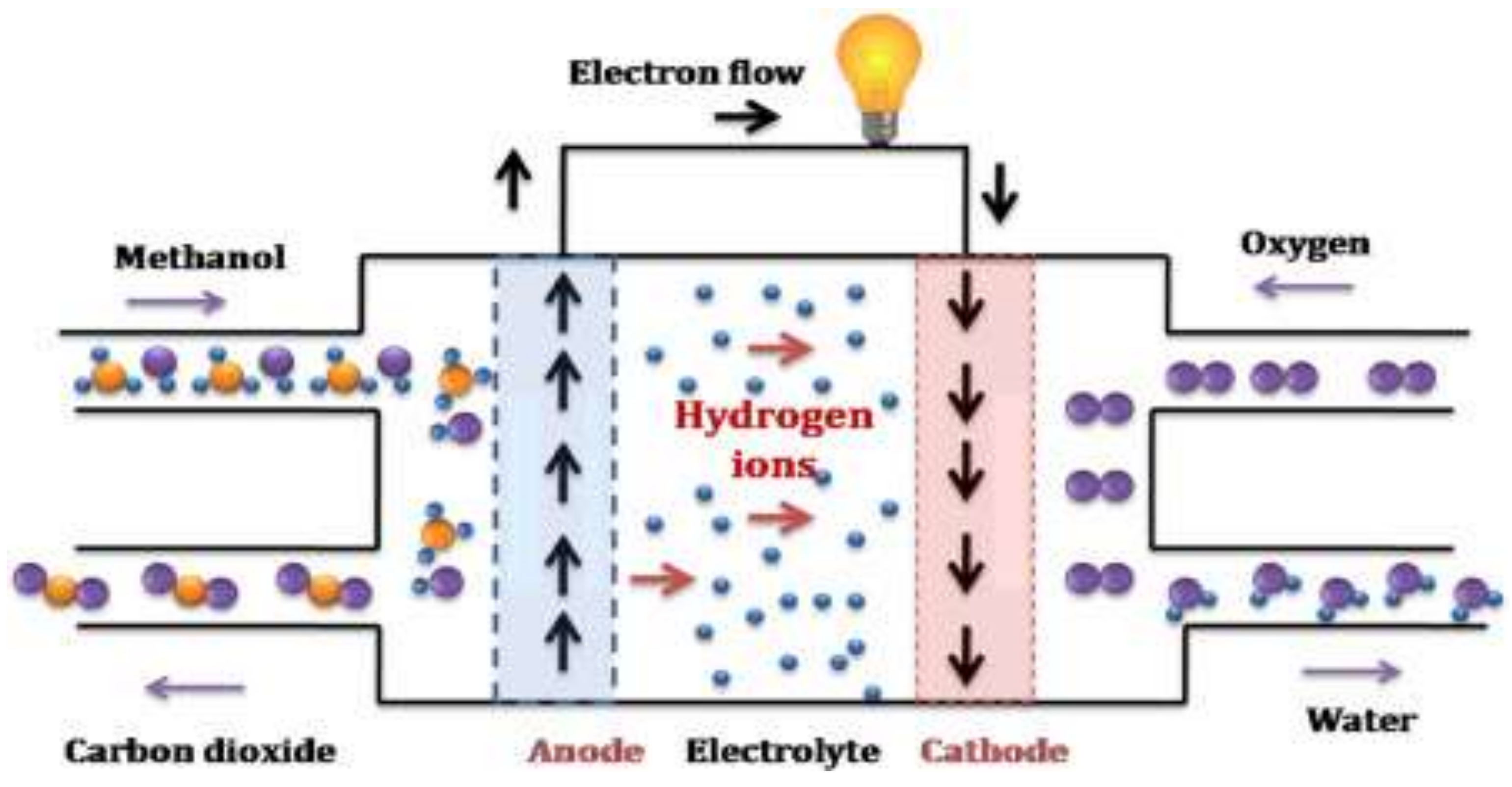
The direct methanol fuel cell dmfc has been considered as the ideal fuel cell system since it produces electric power by the direct conversion of the methanol fuel at the fuel cell anode.
An attempt by matthew feickert to build an operational direct methanol fuel cell for his independent study in chemistry during the 1st semester for the 2006.
High low temperature fuel cells fuel cells are subdivided in low temperature lt and high temperature ht fuel cells.
Lt fuel cells work in a range from room temperature to approx.
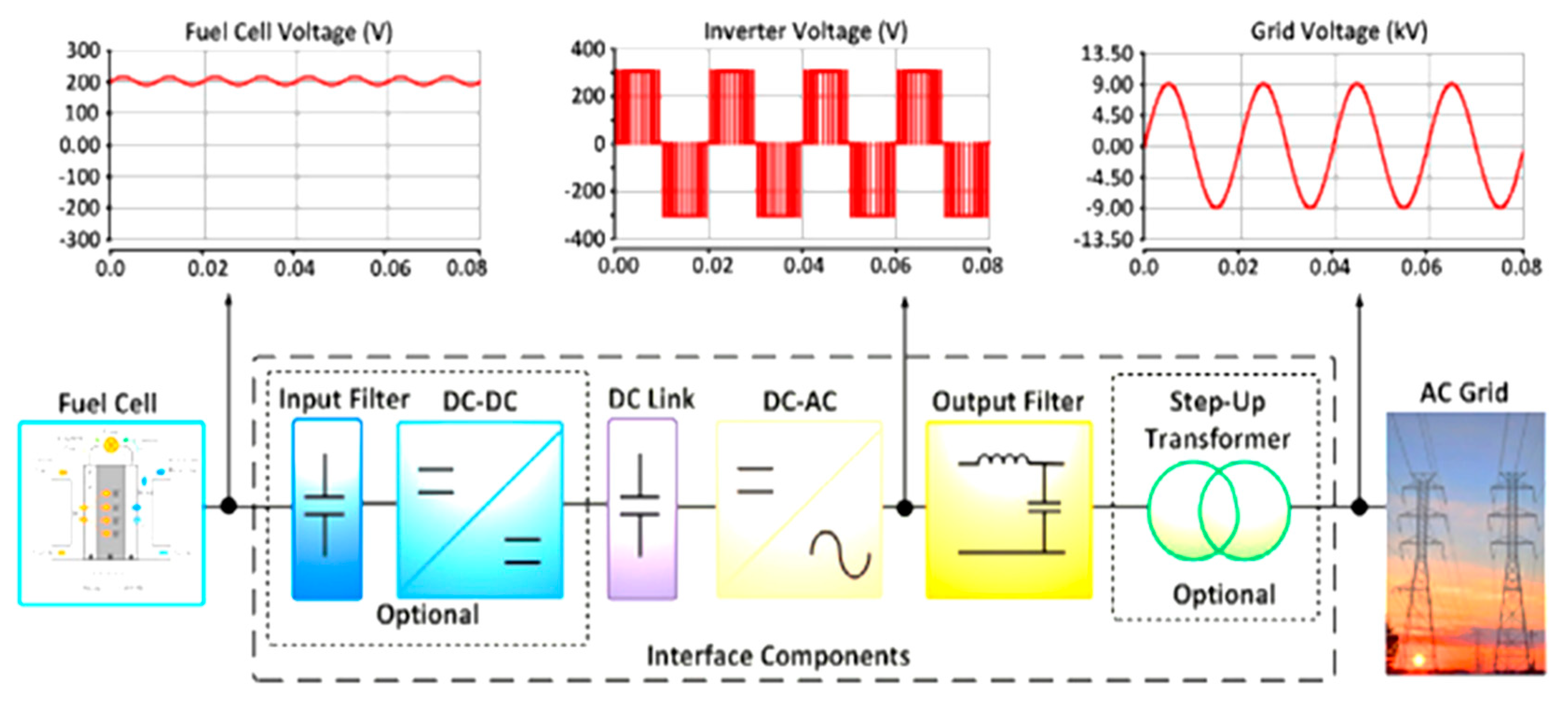
Fuel cell systems have a high efficiency which in principle can outperform those of combustion engines.
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel often hydrogen and an oxidizing agent often oxygen into electricity through a pair of redox reactions.
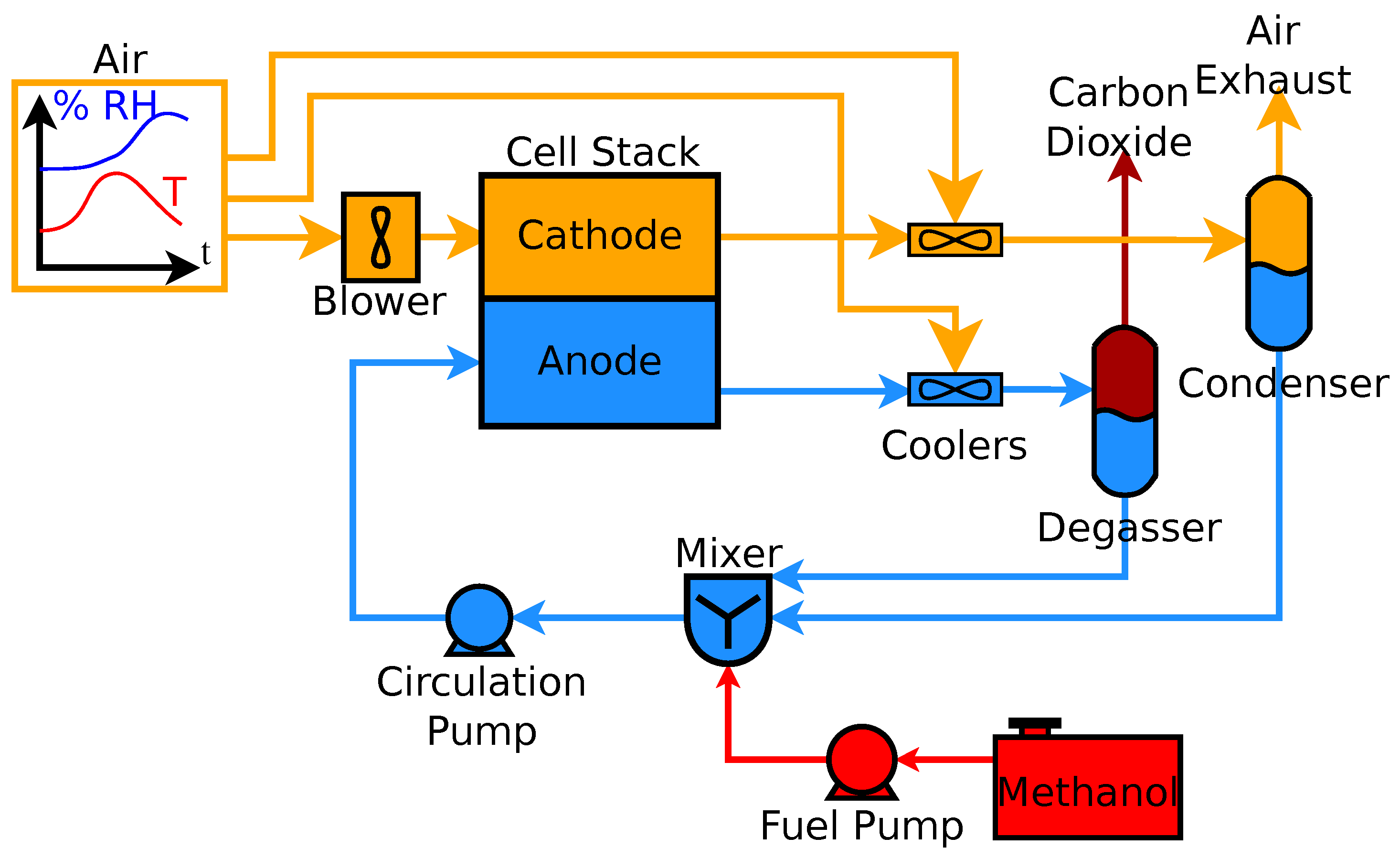
In contrast to indirect methanol fuel cells where methanol is reacted to hydrogen by steam reforming dmfcs use a methanol solution usually around 1m i e.
Common operating temperatures are in the range 50 120 c where high temperatures are usually pressurized dmfcs themselves are more efficient at high temperatures and.
120 c and ht fuel cells from approx.
A direct methanol fuel cell dmfc is an electrochemical cell that generates electricity based on the oxidation of methanol and reduction of oxygen.
About 3 in mass to carry the reactant into the cell.
But real world figures may be only achieved in some years since the development of direct methanol and ethanol fuel cells is lagging behind hydrogen powered fuel.
Bio ethanol based fuel cells may improve the well to wheel balance of this biofuel because of the increased conversion rate of the fuel cell compared to the internal combustion engine.
Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen usually from air to sustain the chemical reaction whereas in a battery the chemical energy usually comes.